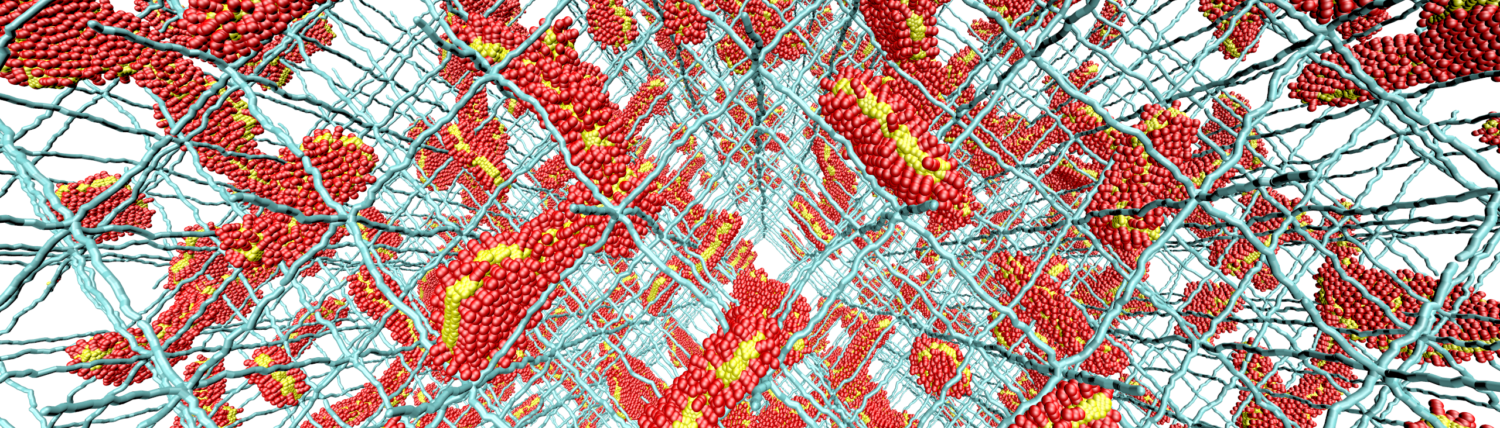
What is common between DNA , all the genetic information in your cells, and plastic shopping bags? Both of these “materials” are composed of long molecular chains that we refer to as polymers. Polymers, whether as constituents of biological or synthetic materials, can share common properties; they can respond to external stimuli, such as mechanical forces, electric fields, flows, or alterations in pH levels or temperature/concentration. They can also transiently adapt geometrical constraints. While type of polymeric soft matter, on one hand, provides unprecedented survival and evolutionary capabilities for life (e.g., the compaction of meters-long DNA in a protective nuclear shell), on the other hand, it creates new opportunities to design next-generation materials and pharmaceutical solutions.
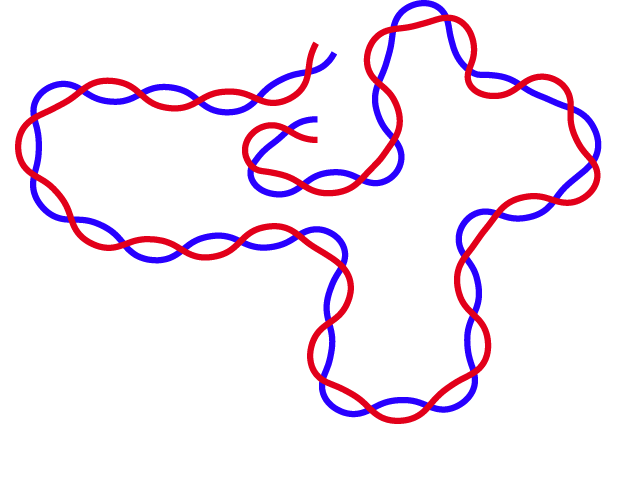
Research in our lab focuses on fundamental problems related to polymers in biological and materials, such as DNA organization and hydrogels, on which we currently have little to no understanding. Specifically, we study the chromosome organization in cells and the potential role of protein-DNA interaction kinetics on this 3D organization . We are also interested in stimuli-responsive properties of polyelectrolyte hydrogels and polymer brushes. In our investigations, we use Molecular Dynamics (MD) simulations at both atomic and coarse-grained levels, along with analytical tools from statistical mechanics.
Research Highlights
- How dng of the genome affect the mechanical properties of the cell nucleus?

The nucleus is more than a vault for DNA — it is also a mechanosensor that resists deformation and transmits physical cues to the genome. In a new modeling study, Attar et al. show that this mechanical resilience hinges on how heterochromatin is connected to the nuclear lamina. Using a coarse-grained polymer simulation of chromatin inside an elastic shell, the authors demonstrate that neither extra heterochromatin nor increased internal crosslinking alone stiffens the nucleus. Only when heterochromatin is tethered to the lamina does the nucleus gain robust, strain-dependent stiffness, with crosslinking providing a secondary boost. Our recent work by our former MA student A. Goktug Attar in colloboration with our collugues from University of Silisia (Poland) and MIT (US) recasts heterochromatin as a mechanically active scaffold whose anchoring to the nuclear periphery underpins nuclear integrity and mechanotransduction — a finding that could reshape how we think about nuclear organization in development, disease and cell migration. Read more on Nucleic Acid Research.
- XX
- XXX